Instant Ice Science Experiment for Kids
This easy instant ice science experiment requires very few

See the Instant Ice Science Experiment in Action
Supplies
- Bottled water
- Glass or ceramic bowl
- Plastic tray or
shallow metal cookie sheet - Ice cubes
- Freezer
- Curious kids

Process
- Put water bottles in the freezer for two hours. (You might want to set a timer to remind you to get them out!) Lay them on their sides for the best results, but try not to dent them.
- Remove the water bottles from the freezer before they freeze. (You’ll know they’re ready when crystals form when you jostle the bottles.)
- Place a ceramic bowl upside down on a flat surface (like a tray) to catch the water overage.
- Place an ice cube on top of the pouring surface.
- Then SLOWLY pour while instant ice forms!
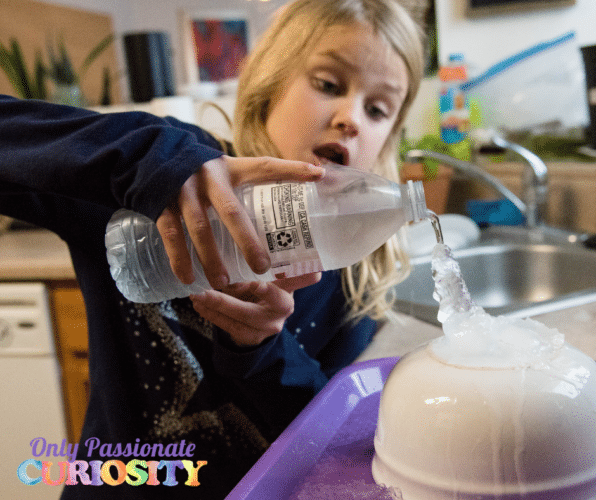
How the Instant Ice Science Experiment Works
This simple but amazing instant ice science experiment is more than just a cool one (see what I did there?). The science behind this experiment lies in the freezing temperature of water and how ice crystals form. This experiment is sometimes referred to as Supercooled Water or Flash Freezing. When the freezing temperature is reached, the water molecules freeze by forming ice crystals.
Why did we put an ice cube on top of the bowl? Because it’s easier for the water molecules to turn to ice on top of already-formed ice crystals. As the ice crystals build on existing ice crystals, they eventually freeze the entire bottle of water.
The process of starting the ice crystals is called “nucleation.” Nucleation starts from an impurity or scratch or piece of dust on the container holding the water. In this case, the nucleation is the water bottle. One ice crystal attaches to the imperfection, and the others grow on top. Isn’t science cool?!
Check for Understanding

Explore this concept further by asking your kids these questions and experimenting:
- Would the experiment work the same if the water had food coloring added to the water?
- Would the results be the same if you started the experiment with hot water in the bottles before you put them in the freezer?
- Does the temperature in the room change the results?
- How high of an ice tower can you pour before it breaks?
Additional Resources
Books
Want to dive deeper into this topic? Check out these fun resources!
The Solid Truth about
Rookie
Videos
Many Kinds of Matter–This jewel of a science book is just the right of information for teaching this topic. Colorful photos and understandable text make this one my favorites!
Why does water expand when it freezes-Naked Science: This fun format uses high-speed drawings and simple text to get the point across. I dare you to try to stop watching!
Brain Stuff–How Can Hot Water Freeze Faster Than Cold Water–All of Brain Stuff’s videos are so well done. You can tell they’re big budget and are pros at this YouTube platform! Enjoy!
Do you love science experiments as much as we do? Then be sure to check out this experiment with salt and ice.










It is so cool ? Tried it with my older sister it was very fun.?